This blog is set up to facilitate learning on:
- The four biological risk groups defined by W.H.O
- The four different levels of biosafety laboratories, its practices and PPE
- The different types of Biosafety cabinets, operations and their maintenance
- About Radioisotope and radioactivity
-Hazards of alpha, beta particles and gamma rediation
- The Handling of radioactive materials safely such as shielding, distance, time, quantity, selection of radioisotopes and management of waste disposal
-Emergency procedures including decomtaminations
Four Biological risk groups defined by W.H.O
Human and animal infectious microorganisms classification is based on pathogenicity of agent, mode of transmission and host range of agent, availability of effective preventative measures and treatments.
· RISK GROUP 1 - a microorganism, or material containing microorganisms, that is unlikely to cause human, plant or animal disease. There is low individual and community risk.
· RISK GROUP 2 - a microorganism, or material containing microorganisms, that can cause human, plant or animal disease, but is unlikely to be a serious hazard to laboratory workers, the community, livestock, or the environment; laboratory exposures may cause infection, but effective treatment and preventive measures are available, and the risk of spread is limited. Includes human opportunistic Pathogens. There is moderate individual risk and limited community risk.
· RISK GROUP 3 – a microorganism, or material containing microorganisms, that usually causes serious human or animal disease and may present a serious risk to laboratory workers. It could be present a risk if spread in the community or the environment, but there are usually effective preventive measures or treatment available. There is high individual risk and limited community risk.
· RISK GROUP 4 – a microorganism, or material containing microorganisms, that usually produces life-threatening human or animal disease, represents a serious hazard to laboratory workers and may be readily transmissible from one individual to another. There is high individual and community risk. Effective treatment and preventive measures are not usually available.
Biosafety Laboratory Level 1 BSL 1 is a basic level of containment appropriate for work on defined and characterized strains of viable microorganisms not known to cause disease in humans. Standard microbiological practices are required with no special primary (safety equipment) or secondary (facility design) barriers recommended, except for a sink for hand washing.
Laboratory facilities
In laboratory design, special attention should be paid to conditions that are known to pose safety problems such as formation of aerosols, work with large volumes and/or high concentrations of microorganisms, overcrowding of equipment, infestation with rodents and arthropods, unauthorized entrance, and workflow. There should be doors for access control. It is not necessary for the laboratory to be designed separate from traffic patterns in the building. Windows that can be opened should be fitted with arthropod-proof screens. The laboratory should be designed to be cleaned easily. Benches should be resistant to water, moderate heat, organic solvents, acids, alkalis or chemicals used to decontaminate the work surfaces.
Personal Protective Equipment
Laboratory coveralls, gowns or uniforms, and gloves must always be worn for laboratory work. Gloves should be discarded aseptically and hands washed after handling of blood, body fluids or potentially infectious agents. Personnel must wash their hands after handling infectious materials and animals, and before leaving laboratory working areas. Safety glasses, face shields (visors) or other protective devices must be worn to protect the eyes and face from splashes, impacting objects and UV radiation. Protective laboratory clothing should be stored in a separate locker from street clothing. Protective laboratory clothing is prohibited outside of the laboratory. Open-toed footwear, food and drinks, cosmetics and contact lenses should not be found in laboratories. Food and drinks should not be stored in laboratory working areas.
Laboratory practices and techniques
Rooms where risk group 2 or higher type microorganisms are handled must have the international biohazard warning symbol and sign (Fig. 1) displayed on it doors. Only authorized persons age 16 and above are allowed in laboratory working areas. Laboratory doors should be kept closed. Animal houses require special authorization for access. Animals not involved in laboratory work are not allowed in the laboratory. “No smoking” “No eating” and “No drinking” signs should be displayed clearly inside and outside the laboratory.

Pipetting by mouth, placing materials in the mouth, and licking of labels is strictly forbidden. Aerosol formation and droplets should be minimized for technical procedures. Biological safety cabinets are used for work with increased risk of aerosolization. The use of hypodermic needles and syringes should be limited to parenteral injection and aspiration of fluids from laboratory animals. All spills, accidents and overt or potential exposures to infectious materials must be reported and a written record maintained. Written records that are to be removed from the laboratory should be protected from contamination. A written procedure for the clean up of all spills must be developed and followed. Contaminated liquids must be decontaminated (chemically or physically) before discharge to the sanitary sewer. An effluent treatment system may be required depending on the risk assessment for the agent(s) being handled.
The laboratory should be free of materials that are not pertinent to the work. Work surfaces must be decontaminated at the end of the working day. All contaminated materials, specimens and cultures must be decontaminated before disposal or cleaning for reuse. Packing and transportation must follow applicable national and/or international regulations.
The laboratory director should ensure that adequate equipment is provided and that it is used properly. Equipment should be selected to take account of certain general principles, i.e. 1) limited contact with infectious material; 2) construction of materials that are impermeable to liquids, non-corrosive, and meet structural requirements; 3) simple operation and ease of maintenance. Performance and construction specifications should be consulted to ensure that the equipment possesses the necessary safety features.
A copy of the safety or operations manual should be available in the laboratory. The laboratory supervisor should ensure the laboratory safety training is provided. When appropriate, there should be an arthropod and rodent control programme. Appropriate medical evaluation, surveillance and treatment should be provided and adequate medical records should be maintained.
Biosafety Laboratory Level 2
BSL 2 is applicable to work done with a broad spectrum of indigenous moderate-risk agents associated with human disease of varying severity. In addition to the guidelines for biosafety Levels 1, the following apply.
Laboratory facilities
Facilities which house restricted agents should have lockable doors. Secondary barriers such as hand washing and waste decontamination facilities must be available. Sinks for handwashing should be equipped with foot, knee, or automatic faucet operation. Eyewash station and autoclave are available. Diagnostic and health care laboratories (public health, clinical or hospital-based) must all be designed for Biosafety Level 2 or above.
Personal Protective Equipment
Primary barriers such as splash shields, face protection, gowns and gloves should be used as appropriate.
Laboratory practices and techniques
Agents with low potential for producing splashes or aerosols can be used safely on open benches. Procedures with high aerosol or splash potential must be conducted in primary containment equipment such as biosafety cabinets. Individuals at increased risk of acquiring infection are limited/restricted from the laboratory area. Immunizations or tests provided for agents in laboratory (hepatitis B vaccine/TB skin testing).

Biosafety Laboratory Level 3
BSL 3 is applicable to work done with indigenous or exotic agents with a potential for respiratory transmission which may cause serious and potentially lethal infection. It is equipped for work with Risk Group 3 microorganisms and large volumes or high concentrations of Risk Group 2 microorganisms with increased risk of aerosol spread. Primary hazards to personnel working with these agents (i.e., Mycobacterium tuberculosis, St. Louis encephalitis virus and Coxiella burnetii) include auto-inoculation, ingestion and exposure to infectious aerosols. Primary and secondary barriers are required to protect personnel and the environment from exposure to infectious aerosols. All laboratory manipulations should be performed in a biological safety cabinet. Secondary barriers include controlled access to the laboratory and a specialized ventilation system that minimizes the release of infectious aerosols from the laboratory. The guidelines for biosafety Levels 1 and 2 apply except where modified below.
Laboratory Facilities
The laboratory should be designed to be separate from unrestricted traffic flow areas within the building e.g. placing the laboratory at the blind end of a corridor, or constructing an anteroom, which is fitted with facilities to separate clean and dirty clothing and a shower. Access to the laboratory area must be through a vestibule, with self-closing and interlockable access doors, and designed to prevent entrance of arthropods and other vermin. Walls, floors, and ceilings should be water-resistant and easily cleaned. A break-through panel may be provided for emergency exit use. Openings in surfaces should be sealed or sealable for decontamination. Air-ducting systems must be constructed. Windows must be closed, sealed and break-resistant. A hand-wash basin should be provided near to each exit door. There must be a ventilation system to ensure proper directional air flow into the laboratory room and air from the laboratory is not recirculated to other areas within the building. Exhaust air must be discharged to the outside of the building through high-efficiency particulate air (HEPA) filters. HEPA filters should permit gaseous decontamination and testing. Biological safety cabinets should be sited away from walking areas and out of cross-currents from doors and ventilation systems. An autoclave for the decontamination of contaminated waste material should be available. Infectious wastes must be transported in sealed, unbreakable and leakproof containers according to national or international regulations. Anti-backflow devices must be fitted to the water supply. Effluents should be decontaminated before being discharged to the sanitary sewer. Facility design and operational procedures should be documented.

Personal Protective Equipment
Laboratory protective clothing must be of the type with solid-front or wrap-around gowns, scrub suits, coveralls, head covering and, where appropriate, shoe covers or dedicated shoes. Front-buttoned standard laboratory coats are unsuitable. Laboratory protective clothing must not be worn outside the laboratory, and it must be decontaminated before use. When appropriate, respiratory equipment must be worn in rooms containing infected animals.
 |
| Gloves |
 |
| Face shield |
 |
| Safety goggles |
 |
| Coveralls |
Laboratory practices and techniques
No individual works alone in the laboratory. The international biohazard warning symbol and sign displayed on doors must identify the microorganism(s) handled and the name of the laboratory supervisor, and indicate any special conditions for entry into the area.
Activities involving infectious materials are conducted in biological safety cabinets, together with other physical containment devices, or special personal protective equipment. A Class III biological safety cabinet may be needed for high-risk procedures involving Risk Group 3 microorganisms. Medical examination is mandatory for laboratory personnel, including a recording of a detailed medical history and a physical examination. A baseline serum sample should be obtained and stored for future reference. Individuals who are immunocompromised should not be employed in facilities with Biosafety Level 3. After a satisfactory clinical assessment, the examinee should be provided with a medical contact card.


Biosafety Laboratory Level 4
The containment laboratory, biosafety level 4, is designed for work with Risk Group 4 microorganisms, dangerous and exotic agents that pose a high individual risk of life-threatening disease, which may be transmitted via aerosols and for which there is no available vaccine or therapy. Agents with close or identical antigenic relationship to Biosafety Level 4 agents should also be handled at this level. Biosafety Level 4 should be under the control of national or other appropriate health authorities.The guidelines for Biosafety Level 3 applies except where modified as follows.
Laboratory facilities
The features of a Biosafety Level 4 laboratory includes
1)A Primary containment system consisting of one or a combination of the following: Class III cabinet laboratory and suit laboratory.
2)Controlled access by placing BSL 4 in a separate building or a clearly delineated zone within a secure
building. Entry and exit is through an airlock or pass-through system.
3)Controlled air system which maintains negative pressure in the laboratory. Supply and exhaust air is passed through HEPA filters.
4)Decontamination of effluents from the suit area, decontamination chamber or shower, or Class III biological safety cabinet using heat. Effluents should be corrected to a neutral pH before disposal. Water from personnel showers and toilets are directed to the sanitary sewer without treatment.
5)Sterilization of waste and materials using a double-door, pass through autoclave, and other equipment for items that cannot withstand steam sterilization.
6)Airlock entry ports for specimens, materials and animals.
7)Emergency power, including dedicated power supply line(s).
8)Containment drain(s).
Personal Protective Equipment
A full-body, air-supplied positive pressure personnel suit is worn.
Laboratory practices and techniques
The two-person rule should apply, whereby no individual ever works alone. A complete change of clothing and shoes is required before and after. Personnel must be trained in emergency extraction procedures. A method of communication for routine and emergency contacts must be established between personnel working within Biosafety Level 4 and support personnel outside the laboratory.
A separate work manual should be developed and tested in training exercises. An emergency programme must be devised with active cooperation with national and local health authorities. Other emergency services should also be involved.


References
http://www.bt.cdc.gov/documents/PPTResponse/table3abiosafety.pdf
http://www.who.int/csr/resources/publications/biosafety/Biosafety7.pdf
http://www.ehrs.upenn.edu/media_files/docs/pdf/biosafetymanual2010.pdf
Introduction to Biosafety Cabinets

Retrieved from
allometrics.com
There are 3 different BSCs ( Class I, II and III) that are specially designed and developed to protect not only the operator but as well as the environment (within the laboratory and/or the external environment) and the product from infectious aerosols and splashes that may be formed during the handling of infectious agent. There are many ways aerosol particles can be created. The transfer of energy to the infectious agent such as stirring, pouring and shaking and activities such as pipetting of infectious agent from one place to another, plate streaking, centrifugation and vortexing can generate aerosols that can adversely affect one’s health via inhalation and also contaminates surface materials. Effective reduction of infections acquired during laboratory experiments as well as contaminations of materials can be achieved when BSCs are used properly.
-Prepare BSC (start of the video)
-Decontamination (0.43min)
-Preparation of work (1.15 min)
- Aseptic Techniques(2 min)
- Cleaning up spills (2.38min)
-Clean up and shut down of BSC (4.33min)
About High Efficiency Particulate Air (HEPA) filters
HEPA filters (Figure 7) filter 99.97% of particles of 0.3um in diameter and 99.99% of particles of smaller or greater size. Microorganisms such as spores, viruses and bacteria can be eliminated from the air using this type of filter. They are usually fixed near the exhaust and supply air systems of the BSCs. Hence ensuring the air discharged from the BSCs is free from microorganisms, especially infectious ones. Careful handling of the filter is essential or it can damage and tear the filter which causes leakage of contaminants into the environment. Maximum operator, environmental and product protection can be achieved with correct configuration of HEPA filters and direction of the airflow.
A) Biosafety Cabinet I
Class I BSC allows operator and environmental protection but no product protection as the product may get contaminated by the unsterilized inflow of air into the work area of the cabinet. The front opening (A) allows entry of arms into the cabinet’s work surface. The sash (B) can be raised to provide more work space for cleaning or other purposes. The opening can be restricted by lowering the sash which results in increased speed of air towards the cabinet. This increases operator’s protection. Some Class I BSCs have integral exhaust fan while others does not hence only relying on the building exhaust fan. Arm-length gloves can be attached to the panel to enhance safety. Class I BCSs can be used to enclose equipments such as centrifuges or small fermenters, or experiments that are more prone to generating aerosols such as culture aeration and cage dumping.
Operation:
1) Unfiltered room air is drawn through the opening (A) below the sash (B) and into the cabinet’s working area. (The inward airflow with a minimum velocity of 75 lfm provides protection of the operator)
2) The circulated air within the cabinet that might have been contaminated by the agents used during the experiment is passed through the exhaust plenum (D) and through the exhaust duct containing the HEPA filter (C).
3) The filtered air is then discharged a) into the laboratory and out of the building via the building exhaust; b) directly outside via the building exhaust; c) directly to the outside.
Figure 1: Schematic diagram of Class I bioafety cabinet. A: front opening, B: Sash, C: Exhaust HEPA filter, D: exhaust plenum
B) Biosafety Cabinet II
Class II BSCs provide operator, environmental and product protection. Product protection is essential during cell and tissue cultures, especially for the propagation of viruses. There are subclasses to Class II BCSs. They are Types A1, A2, B1 and B2. The inflow of room air into the front grill of the cabinet provides operator protection. The presence of exhaust and supply HEPA filter ensures microorganism-free air within the laboratory (Type A1 and A2 BSCs) or discharged to the building exhaust via a canopy. Types B1 and B2 BSCs discharges the air directly to the outside via a hard connection.
Although HEPA are effective in filtering microorganisms and fine particulates however it does not trap volatile gases or chemicals. Hence, experiments involving volatile, poisonous agents should only used Type A2-exhausted or Types B1 and B2 BSCs instead of Type A1 as the exhaust gas is discharged to the outside without recirculating back into the cabinet and the laboratory. However, the amount of these type of chemicals must be limited.
Shows the effectiveness of Class II biosafety cabinet in drawing air into the grills from 34 sec onwards.
A very useful video link about the direction of air in Class II BSCs.
Class II A1 and A2 BSCs:
Build up of potentially harmful chemical vapors in the Class II A1 BSCs (Figure 2) by recirculated air and within the laboratory from the exhaust air can adversely affect the safety and health of the operator hence this type should not use volatile, harmful chemicals. Although Type A1 and A2 BSC can discharge air to the outside however it must be carried out in a manner without disrupting the airflow of the cabinet. Moreover, they should never be connected hard-ducted to the building exhaust systems as inconsistent pressure and volume of air within these systems could not match with the airflow requirements of these cabinets.
Operation:
1) Inflow of room air is drawn through the front grill with a velocity of 75 lfm by an internal fan at the opening of the cabinet (A). Class II A2 BSC’s (Figure 3) operating is similar to A1 BSC with the exception of 100lfm intake air velocity and the plenums are under negative pressure to the laboratory.
2) The supply air is sterilized as it flows through a HEPA filter and is introduced into the cabinet.
3) The filtered air moves downwards, forms a ‘split’ about 6-18 cm from the work surface. Half of the downward air is drawn towards the front grill and the other half towards the rear exhaust grill. Aerosol particles that may be formed during the experiment can be taken up quickly by the downward airflow which is then passed through the front and/or back grill. This ensures product protection.
4) The contaminated air is then drawn into an area between the supply and exhaust filters located at the top of the cabinet via the rear plenum. Due to the different relative size of these filters, about 70% of air recirculates back into the work area of the cabinet through the supply HEPA filter and 30% of the air is discharged into the environment.
Figure 2: Schematic diagram of Class IIA1 biosafety cabinet. A: front opening, B: Sash, C: Exhaust HEPA filter, D: supply HEPA filter, E: rear plenum, F: blower Retrieved from
http://web.princeton.edu/
Class II B1 BSCs:
Class II B1 BSCs (Figure 4) must be hard-ducted to a exhaust system. Small amount of hazardous chemicals such as organic solvents and carcinogens are allowed be worked within the work surface of the cabinet. Since most air is discharged through the rear gill of the cabinet, work involving hazardous agents and vapors should be conducted near that area to ensure these chemicals are discharged to the outside.
Operation:
1) Room air travelling at a minimum inflow velocity of 100 lfm and some recirculated air that is presented in the cabinet are both drawn through the front grill by the cabinet’s supply blower.
2) The influx of air flows upward along the plenum and through the supply HEPA filter.
3) The microorganism-free air flows downwards to the work area through a backpressure plate.
4) The air ‘splits’ half way through as it travels downwards before reaching the flow air.
5) 70% of the down flow air drawn through the rear grill and exhaust exhaust HEPA filter before it is being discharged from the building
6)The other 30% of air is drawn through the front grill and plenum and passed through the supply HEPA filter before recirculating back into the cabinet.
Class II B2 BSCs:
It is a total- exhaust cabinet without any recirculating air. 100% of the air is discharged to the outside. This sub type of Class II BSCs able to provide containment of biological and chemical agents. However, one thing to take note is that some chemicals may erode the filter and/or container hence losing its containment. Working as much as 1200 cubic feet per min to discharge the air completely, it makes Class II B2 BSCs( Figure 5) expensive to operate. Furthermore, better equipments are needed to maintain the high static air pressure that is required for operation hence increasing the cost even more. Therefore, the use of Class II B2 BSCs are used only when necessary.
Operation:
1) At the top of the BSC, the supply blower draws in air from the outside or within the room.
2) The filtered air passes through the supply HEPA filter and flows downwards towards the working area of the cabinet.
3) It is then drawn through both the rear and front grills together with an additional amount of room air that is required to produce a minimum inflow velocity of 100 lfm.
4) The air is then channeled to the building exhaust system and to the outside as it passes through exhaust HEPA filter.
C) Class III Biosafety Cabinet:
The usage of Class III BSC (Figure 6) involves highly infectious microbiological agents. Maximum protection is provided for both the environment and the operator. It is gas-tight containment with a fixed non-opening view window and there are two exhaust HEPA filters compared to the other classes that only have one. Supply air is also HEPA-filtered. Materials are introduced into the cabinet through a dunk tank from the floor of the cabinet or through a double-door pass-through box, for example an autoclave that cab decontaminate materials introduced or taken out from the cabinet. The exhaust system located externally to the BSC maintains the negative pressure within the cabinet (about 124.5 Pa). Long rubber gloves can be attached to the glove ports to allow the movement of materials within the cabinet without physical contact with the materials itself hence providing better protection to the operator.
Operation:
1) Room air is drawn through the supply HEPA filter which is located at the top of the cabinet and into the cabinet.
2) The circulating air within the cabinet is then drawn out to the outside as it passes through the exhaust HEPA filter.
Figure 6: Schematic diagram of Class III biosafety cabinet. A:Glove ports with O-ring for the attachment of arm-length gloves to cabinet, B: Sash, C: Exhaust HEPA filter, D: supply HEPA filter, E: double-ended autoclave or pass-through box. Retrieved from
http://web.princeton.edu/

Figure 8: Class III Biosafety cabinet Retrieved from
http://www.traderscity.com/
The table summarises the differences between each Class of biosafety cabinet.

Table 1: A summary of the different Classes of Biosafety Cabinets with reference to
http://www.cdc.gov/biosafety/publications/bmbl5/BMBL5_appendixA.pdf
ADDITIONAL INFO
There is a lot of things to consider when choosing the suitable BCS. Firstly you have to know how the different BCS works.
· Do the cabinet protect the materials/product?
· What kind of protection needed? Product protection? Protection of operator against the different Risk Groups? Protection of operator against radionuclides and volatile toxic? Or a combinations of these.
· Is there an intention to reduce product contamination? Is it alright if the product gets contaminated because of the inflow of air (which may be contaminated) from the lab to the cabinet? If it is not alright, then BSC I shouldn’t be used cause they don’t provide product protection.
· Are the agents that you are working on volatile? Please note that although the HEPA filter does trap microbes but they do NOT trap volatile agents so even if the recirculated air passes though the filter and into the cabinet again, the air does contain volatile substances.
· Know how the cabinets operate. How the air is being moved and filtered off. How much air is being recirculated back into the cabinet.
· Is the agent that you are using extremely infectious (Risk Group 4)? If that is so, you would want to use a cabinet that does provide EXTRA environmental protection so that when the the air is filtered out into the environment, none of these infectious agent is out into the environment hence increases the level of protection to the community.
Note to ALL: The explanations above is just for easy understanding. It is solely based on my own interpretation with reference to their special characteristics.
Re-enforcement: volatile or toxic chemicals should not be used in BSCs that recirculate air. Examples: Class I, Class IIA1, Class II A2, Class IIB1( acceptable for work with small amount of volatile chemicals and radionuclids.
Class IIB2: can use large amount of volatile and radionuclides chemicals.
General Operation of BSC:
· Lift the sash to a recommended level (Operator seated with the bottom of sash below the level of his/her armpits)
· Minimise the number of materials within the BSCs to reduce air disturbance that may affect the degree of protection covered. It can be done by listing down only the necessary materials required for the experiment
· On the BSC and the blowers for at least 5 minutes before any work as to allow the cabinet to be cleared without any particulates present within the cabinet
· Before work, do check the air intake and exhaust grill if there is any blockage, and the pressure gauge reading.
· Remember to switch on the alarm if the BSC has one.
· Check whether the air flow of the cabinet is correct by placing something light such as a piece of tissue paper above the edge of the cabinet. Make sure it is drawn in.
· Disinfect the surface working area, inner walls of the cabinets as well as the window and sash with 70% ethanol or bleach or other suitable disinfectant.
· Wipe the materials needed with disinfectant before introducing them into the cabinet to reduce contaimination. Arrange the materials and equipment on the surface working area in such a way that the work flows from a clean to contaminated areas. This means that bins are located at one end of the work area.
· It is important to wear protective clothing. Don’t forget to wear appropriate gloves. It is ideal that the gloves are pulled up and over the cuffs of the laboratory sleeves.
· Reduce quick movements of hands in and out of the cabinet as these actions disturb the airflow hence reduces protection. The operator should move the arms in and out of the cabinet slowly and perpendicular to the opening of the BSC.
· Most BSCs operates throughout the entire day.
· BSCs such as Class II A1 and A2 (with exhausting activity to the room or the exhaust ducts) can be turn off when they are not in use.
· Hard-ducted BSCs such as Class II B1 and B2 requires contant airflow through them all the time to maintain the air balance of the room.
· Purge the BSCs after end of work.
· Work as far within the cabinet as possible without compromising your actions and comfort level.
· Open flames within the cabinet are not encouraged as it disturbs the airflow of the cabinet that can compromise safety. Heat can also cause damage to the internal walls of the cabinet as well as HEPA filters.
· Disinfect cabinet after completion of work. Remember to purge the BSC after use.
· Remove protective clothing and gloves and dispose them appropriately. Wash your hands before leaving the room.
Maintenance of BSCs:
1) Changing of HEPA Filters
Ø When filter becomes overloaded that airflow can no longer be maintained
Ø Decontaminate filters before removal via the use of formaldehyde gas
Ø Ensure no gas leakage from the cabinet during decontamination of filters.
Ø Introduction of bag-in/bag-out (BIBO) filter protects the operator and the environment during the handling of filter
2) Certification
Each BSC should be certified according to national or international performance standards to determine the wellness of the operating functions and integrity of cabinets during installation and regular cheak-ups by skilled technicians.
Several tests done by the technician includes:
Ø HEPA filter leaks
Ø The profile of downflow velocity of air
Ø Face velocity of air
Ø The rate of negative pressure
Ø Air-flow smoke pattern
Ø Lighting Intensity Test
Ø UV Lamp Test (some BSCs have UV lamps)
3) Cleaning and Disinfection of work area
Ø Decontaminate surface of work area of BSCs before and after use.
Ø Wipe with a disinfectant such as 70% alcohol or bleach that kills any microorganisms present on the work area.
Ø At the end of the work, the work surface and the sides, back and interior glass
4) Decontamination
Ø Done before filter change and removal
Ø By fumigation using formaldehyde gas
Ø Should be done by skilled technician
References:
3)
http://ors.uchc.edu/bio/resources/pdf/3.2.3.A.3_nuaireBSC.
4)
http://www.med.nus.edu.sg/bch/pdf/safety/SOP%20Safe%20Operation%20of%20Biological%20Safety%20Cabinet.pdf
_____________________________________________________
What are radioisotopes?
Isotopes are atoms with nuclei that have the same number of protons but different number of neutrons. They are elements having the same atomic number but different mass numbers.
With the same number of protons, the different form of element have the same chemical behavior and with different number of neutrons, they have different radioactivity. An isotope that is radioactive is called a radioisotope or radionuclide. Usually, elements have the same proton and neutron number as this makes them stable. However, additional one or two neutrons will cause a shift in binding energy. This binding energy refers to the repelling forces of the like charges of the protons that holds the nucleus together. This is called an unstable atom, and the unstable nucleus gives off radiation that is radioactive. Most atoms are stable, but the unstable atomes have excess energy. to become stable, the atom rids itself of its extra energy by casting it off in a form of electromagnetic waves called radiaition.
All elements with atomic number larger than 83 are radioisotopes with unstable nuclei and are radioactive. They often exists for only minutes and are measured in ' halve lives' which refers to the time to take for half the radioisotope to dissappear.they are defined as isotopes of an element that emits alpha, beta, or gamma radiation during its decay into another element. (A Dictionary of Nursing, Oxford University Press 2008).
What is Radioactivity?
Radioactivity can occur both naturally or artificially. Potassium-40 is an example of a naturally occuring radioisotope that is part of our body. As an unstable nucleus decays, it may give out :
1) an
alpha particle (we use the symbol
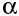
)
2) a
beta particle (symbol
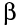
)
3) a
gamma ray (symbol
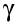
)
1) Alpha radiation
It has the same make up to the nucleus of a helium atom, with 2 neutrons and protons. They have positive charges and little penetrating power.They are heavy and can only cover a short range and are hard to detect because they cannot even penetrate an inch of water, paper, dust or clothing.
2) Beta Radiation
Beta particles have a negative one charge and it is identical to an electron. They are fast and light with intermediate penetrating power and are stopped by aluminium sheets or plastic. They have a range of several feet. Clothing do provide some protection against beta radiation.
3) Gamma radiation
Gamma rays are waves and not particles, which means they do not have mass and a charge. With a high penetrating power, they can penetrate thick sheets of leads or concrete. There are no naturally occuring gamma sources and it isnt actually radiation decary as they do not change the state of nucleus. An example of gamma radiation widely used are x rays. They can travel a long distance through air and can penetrate deep into the human tissue. Gamma rays are usually emitted together with alpha and beta radiation during radioactive decay.
Hazards of Alpha, Beta and Gamma Radiation
Hazards of Alpha Radiation
Due to their positive charges and low velocity, the alpha particles are densely ionizing and lose all of their energy after a short distance. Only the thin layer of dead cells on the skin surface will completely attenuate an alpha beam. Alpha radaitions are of no external hazard. However, if they are ingested or swallowed, they can cause large radiation does to a specific tissue. when ir occurs in the DNA of our cells, its metabolism and activites are disrupted and may even mutate to become cancerous. though they have the lowest penetrating power, they are very dangerous due to their strong ionizing power.
Hazards of Beta radiation
Beta particles covers a longer range than alpha particles but they ionise less strongly. This also means that they have a larger penetrating power and can cause higher external damage to the skin and affect cells inside our bodies.
However, they do have more penetrating power, which means that they can get through your skin and affect cells inside you. They can penetrate living matter and change the structure of molecules. This can be considered as damage if the struck molecule is DNA material that predisposes to a mutation. Beta radiaiton is used in radiotherapy to kill cancerous cells. They can penetrate the layer of cells where new skin cells are produced, and prolonged localized exposure can cause skin injury.
Hazards of Gamma Radiation
Gamma rays do not ionize at all and does not cause damage directly. Gamma rays cannot be controlled and stopped, and lead or concrete clothing is used in order to keep one safe. Once absorbed by an atom, it gains alot of energy and may emit other particles. It carries so much energy it may harm the surrounding viable cells. Gamma rays are very pentrating and exposure may cayse ramage through external diffusion. They are very damaging to rapidly diving cells, and prolonged exposure can lead to burning of the skin and irreversible damage to internal tissue and organs. Chromosomes maybe affected and high levels can even be fatal.
References:
http://www.blogger.com/goog_849083835
http://www.blogger.com/goog_849083835
http://www.bnl.gov/ewms/ser/99ser/appc.pdf
Safe handling of radioactive materials
· 1) Basic lab rules such as no eating & drinking, proper ppe( lab coat, safety glasses, disposable glove)
· 2) Avoid working with open wounds
· 3) Wear double pair of glove and change the outer pair frequently to prevent permeation of the radioactive materials
· 4) Proper labelling, indicating the presence of radioactive materials, on cabinets, containers, doors of lab and anywhere else that the radioactive materials are stored
· 5) Ensure that no unauthorised personnel can have access to radioactive materials by locking up cabinets or the lab itself
6) Use lab instruments to control radioactive materials whenever possible to minimise direct contact as well as provide a distance between the object and the handler
7) Use a radioisotope fume hood when handling volatile radioactive materials
8) Use materials such as lead sheeting or lead glass when handling highly radioactive materials
9) If transportation is needed, double contain the radioactive materials and use a push cart to increase distance between the handler and the materials
Disposal of radioactive materials
· Dispose radioactive contaminated sharps into a radioactive waste container instead of the sharps bin
· Do not use biohazard bags for radioactive waste
· 3 types of wastes: low level, intermediate level and high level
· Disposal of high level waste
· Waste that contains high radioactive compounds
· Waste is stored in multiple layers of corrosion proof materials such as stainless steel and surrounded with bentonite clay to inhibit movement of water.
· Then, it is stored in a stable rock structure and bury deep in the ground to allow the radioactivity to decay for 40 to 50 years
· Disposal of intermediate level waste
· Wastes include resins and chemical sludge.
· Solidify on concrete or bitumen and bury in landfills
· Disposal of low levelled waste:
· Wastes include normal waste such as paper and clothes contaminated with small amounts of short lived radioactive compounds.
· Incinerate in closed containers to decrease volume and bury in landfill
What to do in times of emergency?
There should be a list of emergency contact information and emergency procedures posted at areas where radioactive substances are stored and used and at entrances. Contacts should include radiation safety office (RSO), public safety, environmental health and safety and fire department and ambulance services.This serves as a guideline to what must be done when facing such situation.
In case of emergency, it is important to notify the radiation safety officer and he or she can carry out proper procedure for decontamination and decide on whether to take further actions. It is also necessary to notify all staffs working in the room so that they are aware of the situation. No one is allowed to enter the restricted room or resume their work unless the RSO approves. Always keep calm and evacuate the areas in a orderly manner to prevent any chaos.
For personnel decontamination, affected area should be washed with warm water and soap. Avoid scrubbing the skin as it will cause abrasion which breaks the skin and lead to internal contamination. If there is a wound at the affected area, flush it to remove the contaminants. Seek medical treatment or call 995 if it is fatal. Carefully remove the contaminated clothing and place it in a plastic bag or on absorbent paper. Inform the RSO and administer first aid if injury is serious. The safety of the personnel is the top priority, followed by decontamination.
When dealing with spillage, use absorbent material like paper towels to absorb the spillage to prevent the spillage from spreading. Use a decontamination solution such as RadiacWash or IsoClean or a strong detergent to clean the area with paper towel. Wiping should be done from outward to inward so as to prevent the contaminants from spreading. All waste materials should be properly disposed into a radioactive waste container. Ensure that the clean-up is done while wearing proper protective equipment and gloves.
If there is a spillage of solid radioactive substances, use an appropriate equipment to pick up the substances and dispose them into a radioactive waste container. The following decontamination procedures are similar to the liquid spillage. No personnel are allowed to work in the area until approved by RSO.
For emergencies that involve radiation in the form of dusts or gas, all personnel should evacuate the room immediately and close all windows to prevent the radioactive particles from escaping into the environment. Hold your breath to avoid inhaling the gas. Notify the RSO. Lock the door after leaving the room and place a notice at the entrance to inform people that no one is allowed back into the room until approval is given by the RSO.
If over exposure to radiation is suspected or ingested the radioactive materials, inform the RSO or public safety immediately to seek immediate medical treatment.
Reference





























